Experience
Bridging Engineering Rigor with Human-Centered Product Strategy
With a foundation in Biomedical Engineering and an M.A. in Business Design, I specialize in the intersection of technical precision and user-centric strategy. I move naturally between managing complex product lifecycles—from market research and international expansion to high-stakes Quality Assurance—and translating business logic into actionable engineering goals. My experience across AI-driven health-tech, e-commerce, and social impact platforms has taught me that the best products are built when data-driven systems meet empathetic design.
Vision
To lead the development of intelligent, scalable solutions where technical excellence and human insight align to solve complex global challenges across any industry.
Mission
To empower cross-functional teams to build high-impact products by bridging the gap between engineering, design, and business stakeholders through agile leadership.
Process
Deconstruct complex problems through systems thinking, design the right technical architecture, and execute with rigorous testing to ensure seamless user experiences.
Bridging Engineering Rigor with Human-Centered Product Strategy
With a foundation in Biomedical Engineering and an M.A. in Business Design, I specialize in the intersection of technical precision and user-centric strategy. I move naturally between managing complex product lifecycles—from market research and international expansion to high-stakes Quality Assurance—and translating business logic into actionable engineering goals. My experience across AI-driven health-tech, e-commerce, and social impact platforms has taught me that the best products are built when data-driven systems meet empathetic design.
Vision
To lead the development of intelligent, scalable solutions where technical excellence and human insight align to solve complex global challenges across any industry.
Mission
To empower cross-functional teams to build high-impact products by bridging the gap between engineering, design, and business stakeholders through agile leadership.
Process
Deconstruct complex problems through systems thinking, design the right technical architecture, and execute with rigorous testing to ensure seamless user experiences.
Engineering Mindset Meets Business Strategy: Leading High-Impact Products
Innovatify LTD
A London-based innovation studio building AI-driven digital solutions. As an Associate Product Manager, I played a pillar role in scaling flagship products like HolistiCare and Mia Health. I bridged technical engineering with business logic, managing cross-functional teams across the UK, India, and Portugal to drive product roadmaps and international market growth.

October 2024 – Present
Product Manager & Quality Lead
As a Product Manager for HolistiCare, I served as the operational engine for an AI-powered longevity medicine platform. Reporting to the Senior PM, I orchestrated the daily rhythm of development by leading high-intensity stand-ups and managing 10 Trello boards to ensure strict roadmap alignment. I acted as the primary bridge between clinical practitioners and engineering teams, translating complex biomarker protocols into granular technical requirements. My role was pivotal in maintaining system integrity through rigorous manual testing, where I identified 50+ critical bugs daily to ensure zero regression before deployment. Beyond development, I drove growth by conducting over 60 live demos for global clinics, successfully onboarding 20 B2B clients and managing high-value VIP accounts to inform the next version feature set.
Product Strategy & Agile
QA & Technical Integrity
Growth & Client Success
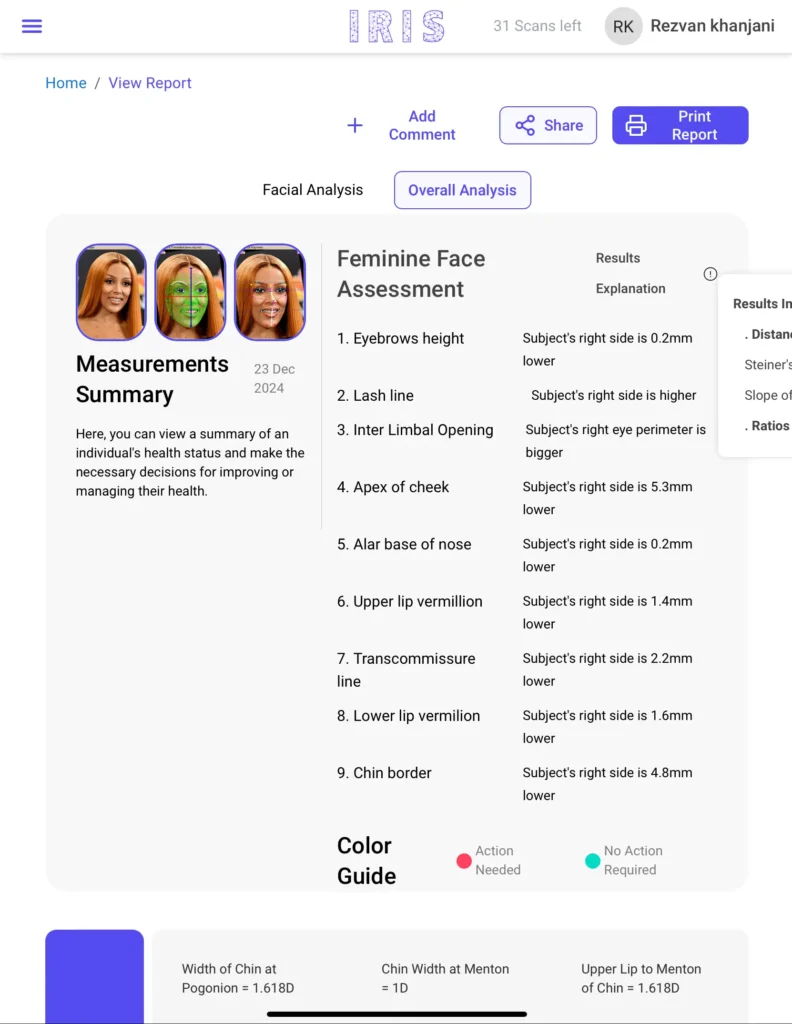
HolistiCare Overview
HolistiCare is a pioneering AI-powered longevity medicine platform designed to scale personalized patient care by analyzing over 800 distinct biomarkers, including blood, genetics, and lifestyle data. During my tenure, the platform transitioned from a complex clinical tool into a high-retention B2B product. My effect was most visible in the professionalization of our delivery and quality standards. I implemented a structured documentation framework using advanced prompt engineering, which reduced manual overhead by 40% and created a repeatable internal wiki for the technical team. By facilitating deep-dive feedback sessions with top-tier longevity doctors, I ensured the development team addressed the most critical clinical pain points, leading directly to measurable engagement growth.
My ability to audit front-end adjustments and benchmark against 9+ global competitors allowed us to maintain a superior UX edge in a rapidly evolving market. I served as the essential link ensuring clinical science and software engineering moved in perfect harmony.





July 2024 – October 2024
Product Manager & Quality Lead
In this dual role representing Innovatify within the Mia Health ecosystem, I served as the central operational link coordinating product development across three international R&D units in Portugal, India, and the UK. I managed cross-functional workflows to ensure localized AI developments—ranging from skin cancer detection to cardiac screening—were integrated into a cohesive product architecture. My responsibilities included spearheading weekly strategic alignment sessions, optimizing global communication latencies, and executing high-stakes product demonstrations for international leads. I acted as a bridge between technical engineering teams and medical panels, ensuring that AI-driven diagnostic tools met strict clinical requirements and user-centric design standards for both European and Asian healthcare markets
Cross-Functional Leadership
Technical QA & Validation
Product Strategy & UX
Mia Health Overview
Mia Health is a mission-driven health-tech firm dedicated to democratizing early health screening through clinical-grade AI that turns smartphones into diagnostic tools. At the time of my tenure, the company was scaling three complex product verticals across disparate geographies: dermatological AI in Portugal, cardiac screening hardware-software in India, and hygiene solutions in the UK.
My involvement was a catalyst for operational synchronization. By implementing structured project management workflows and reducing communication latency between these international units, I achieved a measurable 40% gain in overall system efficiency. My technical rigor in QA—processing over 1,000 test cases—provided the data-driven foundation the engineering teams needed for rapid bug resolution.
Beyond testing, I bridged the gap between medical expertise and technical execution, ensuring that products like "Iris" remained clinically sound while maintaining high usability standards. My contribution transformed fragmented international efforts into a unified, high-velocity product engine.
April 2024 – July 2024
Product Manager & Quality Lead
During the high-growth beta phase of Avatalk, I led the exhaustive daily testing and quality assurance protocols for a web-based platform that redefines professional networking through AI-driven digital business cards. My role was pivotal in ensuring system stability by identifying and documenting an average of 20 unique bugs per day using a structured reporting framework. I collaborated across the technical, design, and marketing departments to prioritize critical blockers and verify that the “AI Twin” user experience remained seamless across mobile and web interfaces. By leveraging tools like Figma and Camtasia, I validated functional flows against high-fidelity prototypes and provided the engineering team with visual evidence to accelerate troubleshooting. My contributions ensured that the platform’s advanced features, such as automated calendar booking and CRM integration, were perfectly aligned with user acquisition and retention strategies.
Cross-Functional Collaboration
Quality Assurance & Stability
Technical Validation & UX
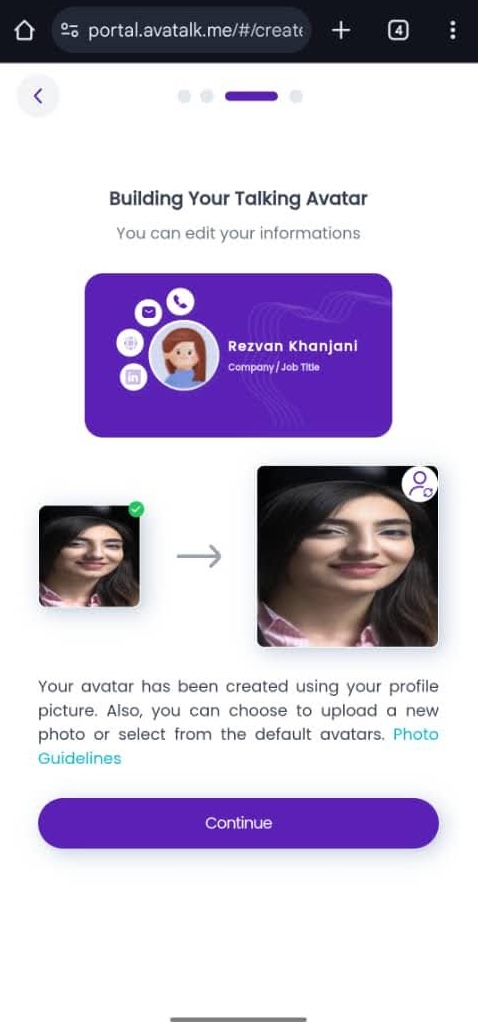
Avatalk Overview
Avatalk is an innovative technology platform designed to modernize professional networking by allowing users to create "AI Twins"—intelligent digital personas that represent them 24/7 without the need for app installation.
At the time I joined, the product was in a high-stakes beta growth phase where system stability and user trust were paramount. My primary impact was the implementation of a rigorous, structured QA framework that significantly reduced the technical debt of the platform. By discovering and triaging 20+ bugs daily, I ensured that critical blockers were resolved within the same development cycle, preventing production bottlenecks during rapid scaling. I acted as a bridge between the creative vision and technical execution, using visual documentation to help engineers understand complex edge cases in the avatar rendering engine.
My work didn't just stop at finding bugs; I actively optimized the user journey by ensuring the product's functional capabilities, such as automated scheduling and social media linking, were robust enough to support aggressive marketing and user acquisition efforts.
Social Innovation at Scale: Redefining Transparency in Philanthropy
Avaye Hamdeli
A social entrepreneurship organization dedicated to empowering women-led families and underprivileged communities through digital transformation. As a Product Strategy Lead and QA Head, I orchestrated the end-to-end development of the Avano platform, transitioning traditional aid into a transparent, data-driven ecosystem. I spearheaded the product roadmap, secured major funding, and implemented rigorous quality standards to bridge the trust gap between donors and social impact.

June 2023 – April 2024
Product Owner
Transitioning from research to lead QA, I headed the quality assurance lifecycle for the “Avano” mobile application, a social entrepreneurship platform designed for radical donation transparency. I was responsible for identifying over 200 distinct bugs during the MVP phase, ranging from back-end logic errors in donation tracking to front-end visual inconsistencies. By implementing a rigorous regression testing protocol, I achieved a 300% reduction in the app’s initial defect count before its soft launch. Beyond technical testing, I collaborated with the UX/UI team in Figma to simplify navigation for non-tech-savvy donors and utilized Prompt Engineering to automate test scripts, accelerating the development cycle. My role ensured that the platform’s high-stakes mission—supporting women-led families and underprivileged communities—was supported by a reliable and intuitive digital experience.
Technical QA & Automation
Product Design & UX Audit
Strategic Impact & Soft Launch
Avano Overview
Avaye Hamdeli is a social entrepreneurship organization, funded by the mining conglomerate Zarrin Maadan Chaf, dedicated to empowering women-led families and distributing medical and nutritional aid. At the time of my involvement, the charity was undergoing a digital transformation to replace opaque, traditional donation methods with "Avano"—a mobile platform for real-time tracking and transparency. My impact was foundational to the platform's reliability and user trust. By discovering and systematically fixing over 200 bugs, I ensured that the critical back-end logic governing financial transparency was flawless before reaching the public. I bridged the gap between complex social mission requirements and technical execution, ensuring the app wasn't just functional, but accessible to the underprivileged populations it serves. My implementation of automated testing and a 300% reduction in defects transformed the development pace, directly contributing to a successful soft launch and a measurable 30% boost in user retention.





August 2022 – January 2023
Product Operations Specialist
In this multidisciplinary role, I applied advanced Biomedical Engineering principles to audit and redesign the nutritional and operational frameworks of a large-scale social aid program. I spearheaded the clinical oversight for high-risk beneficiaries, including the elderly and pregnant women, by interpreting complex lab results to track health outcomes and facilitate specialized medical care. My work involved a complete overhaul of aid package compositions to optimize micronutrient ratios and prevent malnutrition, while simultaneously streamlining warehouse production lines to increase daily output. I served as a critical bridge between clinical data and operational execution, negotiating with suppliers to reduce costs and initiating the charity’s first digital beneficiary needs assessment. My contributions ensured that social impact was driven by technical precision, clinical evidence, and optimized supply chain efficiency
Clinical & Engineering Audit
Operational Efficiency
Product Strategy & Health
Avaye Hamdeli Overview
Avaye Hamdeli is a social entrepreneurship organization dedicated to supporting women-led families and distributing medical and nutritional supplies to underprivileged communities. At the time I joined, the organization was managing high-volume aid distribution but required technical rigor to ensure its health interventions were clinically effective and operationally sustainable. My impact was a total optimization of the aid lifecycle. By applying an engineering mindset to the supply chain, I reduced production costs by 34.5% while increasing warehouse capacity by 25% through reorganized workflows. More importantly, I transformed the "product"—the aid packages themselves—into a precision health tool. I utilized my biomedical background to optimize micronutrient ratios, successfully reducing empty calories by 23% to prevent gestational complications in high-risk mothers. This role proved that data-driven engineering and clinical interpretation could radically enhance the efficiency and biological impact of social welfare programs.



Bridging Traditional Medical Excellence with Digital Innovation
Jarrah Abzar Gharb
One of the most prestigious specialized medical equipment centers in Western Iran, Jarrah Abzar Gharb (“Western Surgical Tools”) boasts over 35 years of operational history. As a primary contractor for major medical universities and hospitals, the firm specializes in the import and distribution of high-stakes surgical equipment and orthopedic implants under strict government regulation. My role focused on spearheading the company’s end-to-end digital transformation, translating decades of offline supply chain expertise into a high-performing e-commerce architecture and a modernized B2B operation.

January 2022 – August 2022
E-commerce Product Specialist
In this role, I spearheaded the comprehensive digital transformation of a leading medical equipment distributor, managing the end-to-end launch of their first official e-commerce platform. I led the project from beta testing to full operational status within a strict 3-month timeline, overseeing both technical development and content strategy. My responsibilities included managing high-stakes procurement and distribution of specialized orthopedic implants in strict compliance with government regulations. I acted as the primary liaison between government regulators, hospital procurement departments, and surgeons, ensuring the seamless delivery of life-saving equipment directly to operating theaters. By bridging the gap between traditional supply chain operations and digital commerce, I successfully opened new B2B and direct-to-consumer channels that significantly expanded the company’s market reach.
Digital Transformation & UAT
Supply Chain & B2G Operations
Sales Strategy & CRM
Jarrah Abzar Gharb Overview
Jarrah Abzar Gharb is one of Western Iran’s largest and most prestigious specialized medical equipment centers, with over 35 years of history in importing and distributing super-specialized medical gear. As a primary contractor for Kermanshah University of Medical Sciences, the company operates under strict IFDA licenses.
My arrival marked a critical shift from traditional distribution to digital-first operations. By spearheading the firm's first e-commerce platform, I didn't just build a website; I re-engineered the company's workflow, resulting in a 60% improvement in order processing efficiency. I successfully digitized a massive catalog of over 700 products, making high-stakes surgical equipment more accessible through new B2B and D2C channels.
My strategic management of the "Virtual Warehouse" and regional partnerships for brands like Osveh directly translated into a 40% revenue boost and a 45% increase in brand-specific sales.


Building the Foundation: Where My Engineering Rigor First Met International Business Strategy.
Chakad Teb Adrian
A pioneering, knowledge-based medical engineering firm founded by the Isfahan University of Technology faculty. Specialized in the design and localization of high-performance rehabilitation technology, Chakad Teb Adrian (“The Peak of Medical Science”) is renowned for creating advanced carbon fiber prosthetic limbs that rival top-tier international standards. Holding prestigious IMED and European Union (CE) certifications, the company serves a global mission to restore human mobility through cutting-edge biomechanical engineering and strategic international market entry.

July 2021 – January 2022
International Product Specialist
In this role, I spearheaded the international market entry strategy for a high-tech medical engineering firm specializing in carbon fiber prosthetic limbs and rehabilitation gear. I orchestrated and executed over 20 in-person product demonstrations and technical presentations for international health delegations, specifically targeting Iraq and Saudi Arabia. As the lead English-language communicator, I translated complex biomechanical engineering concepts for foreign stakeholders and developed all technical presentation materials. I managed high-stakes presentations for major government bodies, including the Martyrs Foundation, resulting in a 50% increase in domestic product sales. My responsibilities spanned the full product marketing lifecycle, from launching a 10-page bilingual corporate website to ensuring all technical documentation met European Union (CE) and IMED certification standards. I acted as a critical bridge between engineering teams and non-technical clients to secure buyer confidence.
Global Market Expansion
Product Marketing & Design
Strategic Partnerships & QA
Chakad Teb Adrian Overview
Chakad Teb Adrin is a pioneering knowledge-based medical engineering firm specializing in the design and manufacturing of advanced rehabilitation equipment and high-performance prosthetics. Renowned for its "Chakad 2 Carbon Paw," which rivals top-tier international competitors, the company operates under European Union (CE) approval and IMED certification. My tenure was defined by transforming the company’s regional presence into an international contender. By professionalizing their English-language outreach and technical documentation, I successfully opened doors to Iraq and Saudi Arabia, securing five major contracts that grew total company revenue by 30% in just six months. Domestically, my management of high-stakes presentations for organizations like the Martyrs Foundation led to a 50% surge in sales. I brought a rigorous QA mindset to the marketing department, ensuring the launch of their bilingual platform was technically sound while bridging the gap between biomechanical engineering theory and client-facing business value.


-
Product Management Translate complex logic into technical requirements for AI-driven platforms and cross-functional teams.
-
Biomedical Engineering Apply rigorous systems thinking to technical compliance and high-stakes clinical data analysis.
-
Business Design Architect resilient models and navigate strategic pivots using M.A.-level Entrepreneurship strategy.
-
Quality Assurance Lead high-stakes UAT and bug lifecycles to ensure zero-regression in global digital products.
-
Operational Strategy Synchronize global teams to streamline workflows and drive international B2B market expansion.